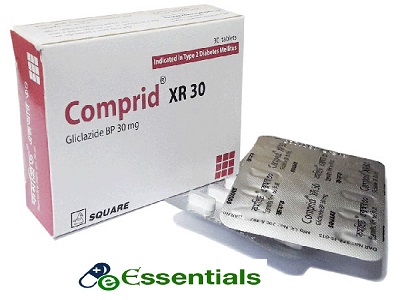
Comprid XR 30mg 10pcs
৳ 60.20
Brand : Comprid XR
Manufacturer : Square Pharmaceuticals Limited.
Indications
Gliclazide is indicated for control of blood glucose in patients with non-insulin dependent diabetes mellitus (Type-II, maturity onset diabetes mellitus) whose hyperglycemia cannot be controlled by diet alone.
Therapeutic Class
Dosage & Administration
Adult: The usual initial dose of Gliclazide is 40 to 80 mg daily, gradually increased, if necessary up to 320 mg daily until adequate control is achieved. A single dose should not exceed 160 mg. When higher doses are required it should be taken twice daily, according to the main meals of the day. For extended release tablet the initial recommended dose is 30 mg daily, even in elderly patients (>65 years); the daily dose may vary from 30 to 120 mg taken orally, once daily.
Gliclazide extend release tablet should be taken with food because there is increased risk of hypoglycemia if a meal is taken late. It is recommended that the medication be taken at breakfast time. If a dose is forgotten, the dose taken on the next day should not be increased. Dose titration should be carried out in steps of 30 mg, according to the fasting blood glucose response. Each step should last for at least two weeks. Gliclazide extend release tablet should be neither broken nor chewed. Gliclazide extend release tablet 30 mg, can replace Gliclazide 80 mg tablets for doses of 1 to 4 tablets per day.
Elderly: Plasma clearance of Gliclazide is not altered in the elderly and steady state plasma levels are similar to those in adults under 65 years. Clinical experience in the elderly shows that it is effective and well tolerated.
Children: Gliclazide as with other sulfonylureas is not indicated for the treatment of juvenile onset diabetes mellitus.
Interaction
Contraindications
Side Effects
Pregnancy & Lactation
Pregnant Women: Gliclazide should not be used in pregnant women although animal studies of Gliclazide have not shown any teratogenic effect.
Nursing Mothers: This drug is contraindicated when breast feeding.
Precautions
Overdose Effects
Symptoms: Hypoglycaemia with or without coma, convulsions or other neurological disorders.
Management: Carbohydrate intake, dosage adjustment and/or change of diet may be helpful. Admin rapid IV inj of concentrated glucose soln for hypoglycaemic coma.
Use in Special Population
Elderly: Plasma clearance of Gliclazide is not altered in the elderly and steady state plasma levels are similar to those in adults under 65 years. Clinical experience in the elderly shows that it is effective and well tolerated.
Children: Gliclazide as with other sulfonylureas is not indicated for the treatment of juvenile onset diabetes mellitus.
Storage Conditions
Brand
Square Pharmaceuticals Ltd